Equine Embryo Transfer Discussed
Embryo transfer (ET) is the most widely practiced assisted reproductive technology (ART) in the mare. This is not a new technology, the first successful equine embryo transfer being reported in 1972, yet limitations set by breed registries slowed its adoption by mare owners. Mares cannot be as successfully superovulated (aka, ovarian hyperstimulation) adding yet another limiting factor preventing its widespread application. For example, a donor dairy or beef cow can be superovulated with hormonal stimulation to produce 10-12 embryos (sometimes more) per transfer cycle. A donor mare under the optimal ovarian hormonal stimulation will only produce 2-3 embryos (many times less) per transfer cycle.
Reasons for the commercial application of ET include: 1) obtaining foals from donor mares during their athletic or performance years; 2) multiple foals from individual donors during a given season or year; 3) foals from young maiden mares; 4) foals from mares with debilitating health problems (e.g., chronic laminitis, fractures, etc.) and lastly, 5) foals from reproductively unsound mares. ET was initially proposed as a promising method for obtaining foals from aged mares (e.g., late teens to early twenties). Clinical trials and more rigorous research experiments have documented that many embryos produced by aged mares are inherently defective. Embryos from such mares have low survival rates after transfer to younger recipient mares; therefore, aged mares are not optimal donor candidates for ET.
The donor mare and one or more recipient (aka, surrogate) mares should be examined thoroughly prior to their selection. If abnormalities are identified in the donor that warrant treatment (e.g., bacterial or mycotic endometritis), appropriate therapy should be initiated before embryo transfer procedures are performed. Selection and management of recipients is equally important, and will assuredly affect the success of an ET program. Recipients should have normal estrous cycles and be free of uterine abnormalities. Their optimum age should be less than 12-14 years, and preferably have had at least one prior foal.
Synchronizing estrus between donor and recipient mares can be accomplished with hormonal protocols using prostaglandin (PGF2a) alone or in combination with exogenous progesterone. Synchrony between follicular ovulations of the donor and recipient(s) is the goal; recipient(s) can ovulate 1-2 days prior to and up to 3-4 days after the donor.
Embryo recoveries have been successfully performed from donor mares bred with frozen, extended-cooled transported, and freshly collected semen. Regardless of the breeding technique used for the donor, it is paramount to pinpoint ovulation time to within a 12 hour window. Embryos are selectively transported through the oviduct into the uterus by day 5 or 6 post- ovulation, at which time they are potentially available for retrieval from the uterus using a simple flushing technique. They can be recovered over the range of day 6 to 9 post-ovulation, with the optimal time of collection between day 7.5 to 8.
The procedure for embryo flushing is very similar to the uterine lavage technique often used to treat a uterine infection (endometritis). The mare is restrained in stocks, and her perineal area is cleansed with a mild detergent, rinsed thoroughly with clean water and dried. The operator introduces a sterile balloon-tipped catheter using accepted technique. After entering the vagina, the catheter is passed through the cervix and into the uterine body. The balloon tip is inflated with sterile saline and is positioned back against the cervix to prevent loss of fluid. Once the catheter is seated appropriately, the uterus is flushed three to four times with an appropriately warm (37.5C) embryo flushing fluid. The uterus is filled with 1-2 L during each flush (3 to 5 L total). After filling the uterus, the flush fluid is allowed to flow back out through the catheter and is passed through an embryo capturing filter. The fluid passing through the filter is collected to monitor its recovery for volume and clarity. Before the final flush, the uterus is massaged per rectum, to aid suspension of the embryo(s) in the medium and enhance fluid recovery. The majority (>90%) of fluid infused into the uterus should be recovered, and it should be free of cellular debris or blood. Recovery of cloudy fluid may indicate the mare had an infection at the time of the flushing attempt, and warrants further diagnostic evaluation. After the flushing procedure, the embryo capture filter cup can be directly examined under a dissecting or stereo- microscope to ascertain recovery of one or more embryos. When an embryo(s) is identified, it is washed by transferring it sequentially through at least three wells of an appropriate embryo holding medium. The embryo is then examined and graded for quality on a scale of 1 (excellent) to 4 (poor).
Embryos can be handled using a 0.25 or 0.5 mL semen freezing straw or 25 μL glass capillary pipette attached to an appropriate syringe. Anytime an embryo is drawn into a handling instrument, the medium containing the embryo should be surrounded on each side by an air bubble and fluid medium. This prevents the embryo from accidentally being wicked out of the instrument should the tip touch something absorbent. The process of picking up and depositing an embryo should be observed under the microscope.
Transfer of the embryo should take place as soon as possible after collection, as it has been shown that pregnancy rates drop if the embryo is out of the mare for more than 1-2 hours. Non- surgical transfer (transcervical) is easy to perform, with pregnancy rates between 60-70%; success being related both to the skill of the operator as well as the quality of the embryo. It is performed smoothly and rapidly, using a technique similar to a routine artificial insemination (AI). A newer approach for embryo transfer using transvaginal ultrasound-guided intrauterine injection of the embryo has recently been described, the initial data indicating a 78% success rate in establishment of pregnancy (18 pregnancies out of 23 embryos transferred).
A technique for cooling equine embryos has been developed, which is a practical method of short-term (up to 24 hr) storage and transportation. This allows the embryo to be collected on the donor mare’s farm and shipped to a distant facility for transfer to a suitable recipient mare. The ability to transport cooled embryos provides practitioners with the opportunity to offer embryo transfer services without the need to maintain their own recipient mare band. While awaiting packaging for transport, equine embryos are tolerant of temperatures between room temperature (25C) and body temperature (37C). Efforts should be made to prevent rapid or extreme changes in temperature. Equine embryos are cooled and transported using defined nutrient media. The packaged embryo can be shipped in an Equitainer, often used for shipping stallion semen; this passively cools the embryo to 5C. Using this system, embryos can remain viable for at least 24 hours, during which time they can be transported via commercial airline or priority overnight delivery to the facility where recipient mare(s) have been prepared and waiting the transfer.
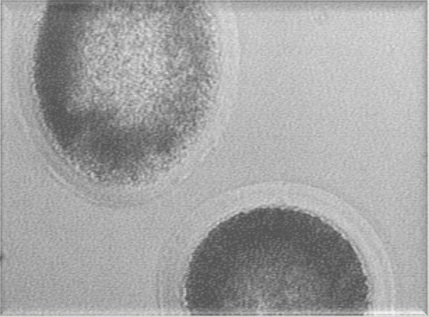
Mammalian embryos have been successfully frozen and stored since 1972 when live mice where obtained after the transfer of freeze-thawed morulae. In humans, the first birth from a frozen embryo occurred in 1984 in Australia. This cryotechnology, derived from rodent and cattle routines, became a necessary part of in vitro fertilization (IVF) programs in order to avoid the risks of multiple pregnancies following the transfer of large numbers of embryos. At present, embryo freezing is a widespread, routine procedure in human infertility clinics. Freezing of equine embryos is not as simple as in the bovine or human, probably due to the fairly high lipid content of equine embryos, and their relatively large size. Best results for freezing have been achieved using small equine embryos (< 300 μ diameter). It is postulated that the thickness of larger equine embryo’s capsule interferes with cryoprotectant permeability. Regardless, it can be performed, and equine embryos are frozen for later transfer, with a success rate for pregnancy of between 30-40%.
Costs associated with application of this ART range dependent upon multiple factors:
1) whether or not the donor mare owner also has his or her own recipient mare or mares available; if not they will have to either purchase or lease one or more recipient mare for use as surrogate(s).
2) whether or not a superovulation attempt will be made with the donor mare; this can add anywhere from $500-$1,000 to the costs of the procedure
3) method of breeding the donor mare with frozen semen breeding typically being more expensive to perform, as well as less likely to induce conception when compared to freshly collected and inseminated semen
4) whether any cooled-transport or cryopreservation of recovered embryo(s) will be performed.
Minimally the mare owner can expect to invest at least $2,000 in the ET process per donor breeding cycle with no guarantee for success when they provide their own recipient mare. The estimated range in the US is expected to be from $5,000 to $7,500 when a leased recipient mare is used. Quite often the costs can and do overrun the estimate.
William B. Ley, DVM, MS, Diplomate, American College of Theriogenologists
Dr. William B. Ley DVM, MS, DACT Articles
Related Links
Allowed: 64M/67108864KB.
Current: 5318KB. Peak: 5388KB.