Equine Viral Arteritis
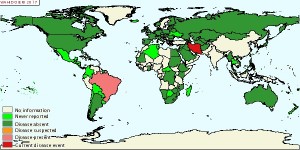
Equine Viral Arteritis (EVA, EAV) is a contagious viral disease of horses whose etiologic agent is an RNA virus in the Arteriviridae family (formerly a pestivirus in the Togaviridae family). EVA is associated with the following clinical signs: pyrexia, depression, conjunctivitis, depression, rhinitis, ocular and nasal discharges, dependent edema, urticaria and late-term abortion in pregnant mares. However, in the majority of cases, EVA infection is subclinical and even horses showing clinical signs experience little mortality. This virus is spread venereally during live cover and in chilled and frozen semen, via aerosolized respiratory tract secretions, in utero and via fomites. The main source of EVA virus is carrier stallions that shed viral particles in their semen. Susceptible mares only shed EVA during the acute phase of the infection, after which they seroconvert and do not become carriers of the virus. Therefore, in order to control EVA in the breeding population, the issue of carrier stallions must be addressed.
The only approved EVA vaccine in this country is ARVAC manufactured by Zoetis. Use of this vaccine is controversial for two main reasons: it is a modified-live vaccine (MLV) so the potential to revert to virulence is present and, the current methods of testing serum samples (virus neutralization test, complement fixation test and ELISA) cannot distinguish between a viral antibody titer and a vaccine-induced antibody titer. Therefore, concerns over vaccination protocols in stallions exist. On the positive side, proper vaccinations of seronegative stallions will decrease the number of carrier stallions, and therefore the incidence of EVA and subsequent abortion storms in mares. On the negative side, any vaccinated stallion will test positive for EVA. This is of concern because EVA is a reportable disease in the USA and several foreign countries deny entry of both seropositive horses and their semen.
A breeding stallion may be vaccinated against EVA, but only after an EVA seronegative titer has been recorded by a licensed and USDA accredited veterinarian. Once vaccinated, boosters need to be given every year at least 28 days prior to breeding (either by live cover or artificial insemination). The reason for waiting 28 days before breeding the stallion, although a carrier state has never been identified in an initially seronegative stallion vaccinated with a MLV, is that following vaccination small amounts of vaccine virus may be shed. Non-pregnant mares should also be vaccinated against EVA and isolated from horses that are seronegative for at least 21 days; however, mares do not require revaccination, as life-long immunity is stimulated with vaccination. The reason for isolating mares for 21 days after vaccination is two-fold: this period of time is required for a mare to build up adequate protection from the virus following vaccination and, there may also be a minimal risk for her to transiently shed vaccine virus. The minimum isolation time-period of 3 weeks is chosen for both mares and stallions because experimentally EVA can be recovered from secretions for up to 3 weeks following infection.
September 2000
Dr. William B. Ley DVM, MS, DACT Articles
Related Links
Allowed: 64M/67108864KB.
Current: 8487KB. Peak: 8556KB.